
January 2025
Psychedelics - A Trip to a Brighter Future
The FDA’s rejection of Lykos Therapeutics’ MDMA in 2024 marked a period of upheaval for drug developers and investors in the psychedelic space. However, the industry should remain optimistic—there is a rich pipeline of agents that could address the clinical concerns that have plagued the field and Spravato’s commercial success provides a launch roadmap for future psychedelics.
The promise of transformational efficacy and improved safety of psychedelic agents in the pipeline, plus the commercial success of Spravato, signal a brighter future for psychedelics.
Bright Spots Emerging in the Psychedelics Field
The FDA’s rejection of Lykos Therapeutics’ MDMA in August 2024 marked a period of upheaval for drug developers and investors in the psychedelic space. However, the field should remain optimistic with a rich pipeline of 80+ agents, including several demonstrating positive clinical data and impending trial readouts.
Enthusiasm for psychedelics is largely driven by the onset of efficacy, durability, and remission rates that are superior to conventional treatments, including the first approved psychedelic, esketamine (Spravato).
- 15+ agents in Ph 2 or Ph 3 have demonstrated one or more of these benefits in clinical trials.
- Such agents could lower resource burden for clinics administering Spravato, especially with the potential for one-time administration.
Heightened concerns about the cardiovascular risks, psychoactive side
effects, and abuse potential exist — these are predominantly linked to the
serotoninergic activity.
- To improve safety, non-serotoninergic mechanisms and alternative analogs are being explored.
- While a REMS program will likely still be required, these benefits may minimize monitoring time post administration and/or improve drug scheduling.
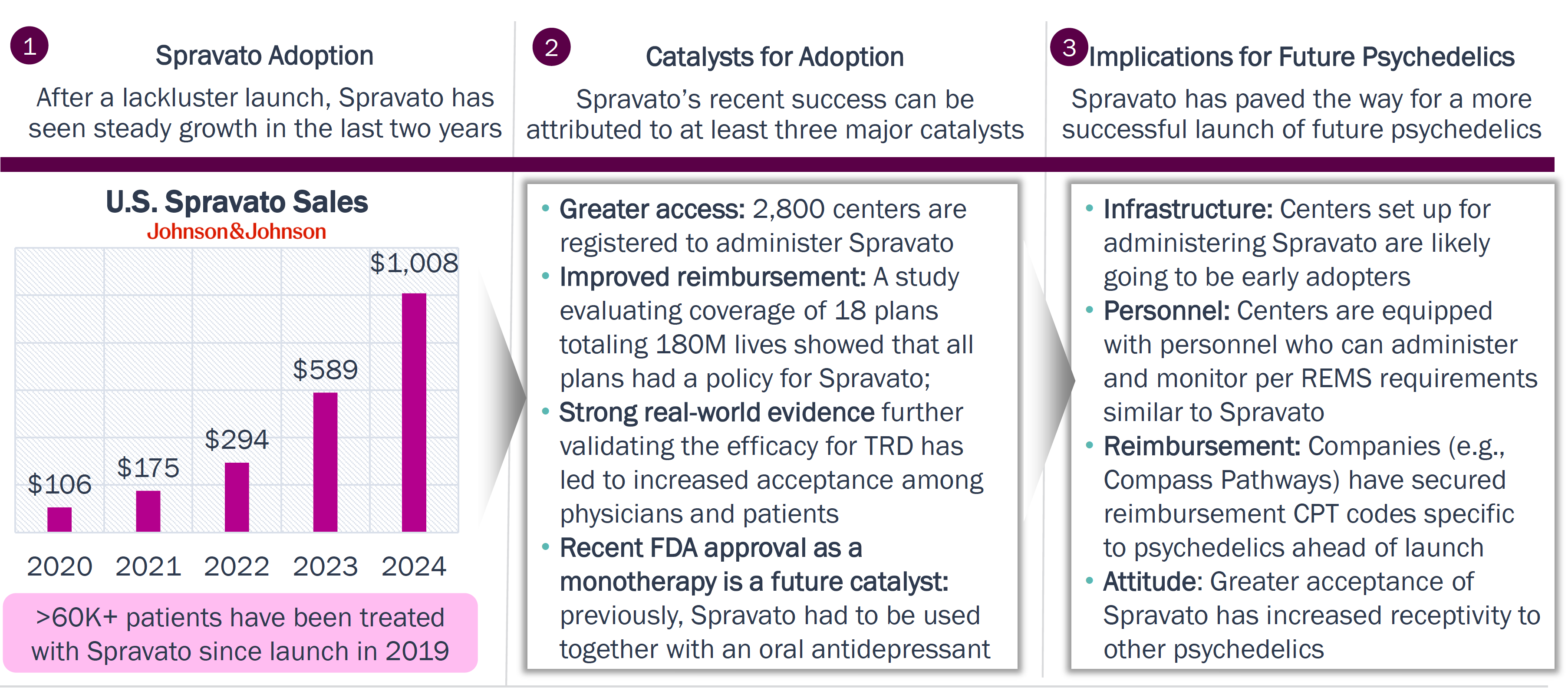
Spravato is a trailblazer for future psychedelics; it set up the infrastructure of target clinics, established a precedent for reimbursement, and brought greater acceptance of the drug class in general.
REMS—Risk Evaluation and Mitigation Strategies. A REMS program is required by the FDA for certain drugs with serious safety concerns and may entail multiple requirements including extra training by providers, monitoring post-administration, and AE reporting
Current Climate
Despite the high-profile failure of Lykos that has sobered the field on the prospect of psychedelics, the field is still ripe with promising agents, including assets in Phase 3 trials
Psychedelic Pipeline
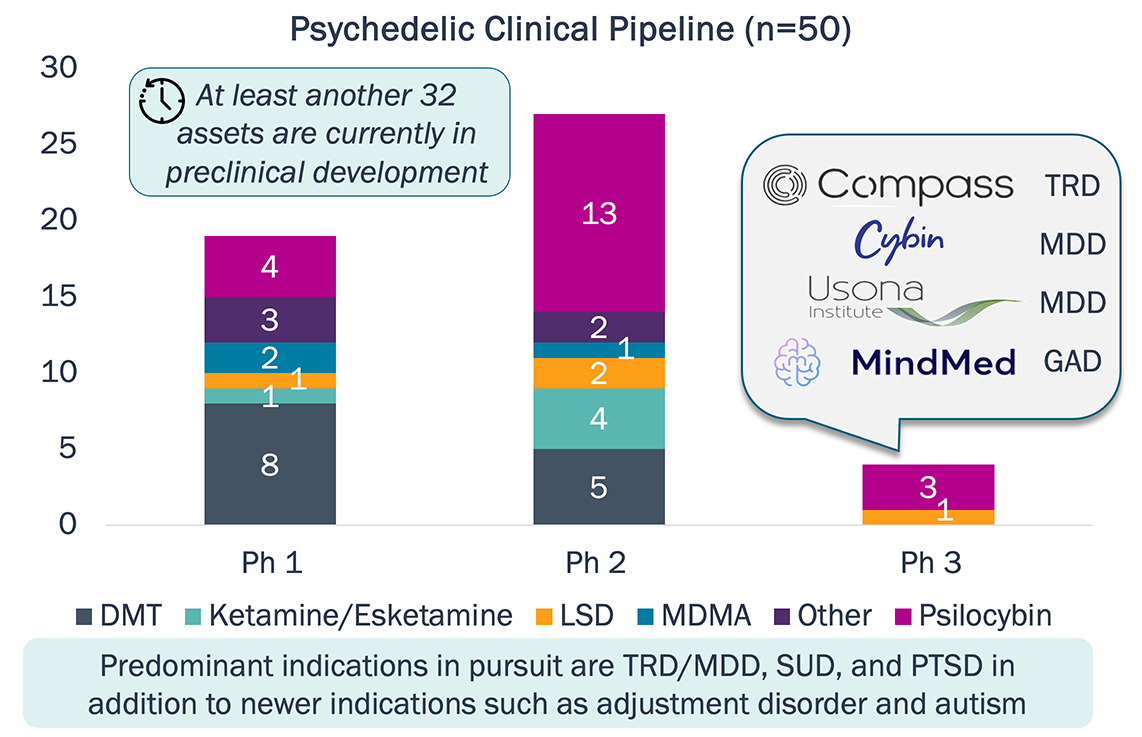
As Lykos developed MDMA-assisted therapy for PTSD, emerging challenges sparked the FDA’s request for an additional Phase 3 study:
-
Confounding concerns of efficacy:
- Functional unblinding due to the psychoactive nature of the drug
- Concerns about a lack of standardization and impact of psychotherapy
-
An incomplete view of safety:
- Questions about the drug abuse potential and lack of data surrounding euphoria with treatment
- The need for comprehensive data on cardiovascular risks, hepatotoxicity, and a plan for a REMS program
TRD—treatment-resistant depression.
MDD—major depressive disorder.
GAD—generalized anxiety disorder.
SUD—substance use disorder.
PTSD—post-traumatic stress disorder.
Other includes
mescaline and proprietary unspecified molecules.
Sources: PharmaProjects; company websites
Efficacy Potential
Psychedelics are psychoplastogens with a greater efficacy potential than traditional treatments, largely due to rapid onset of efficacy, effect size and sustained duration of effect
Relative Efficacy of Psychedelics Compared to Conventional Treatments
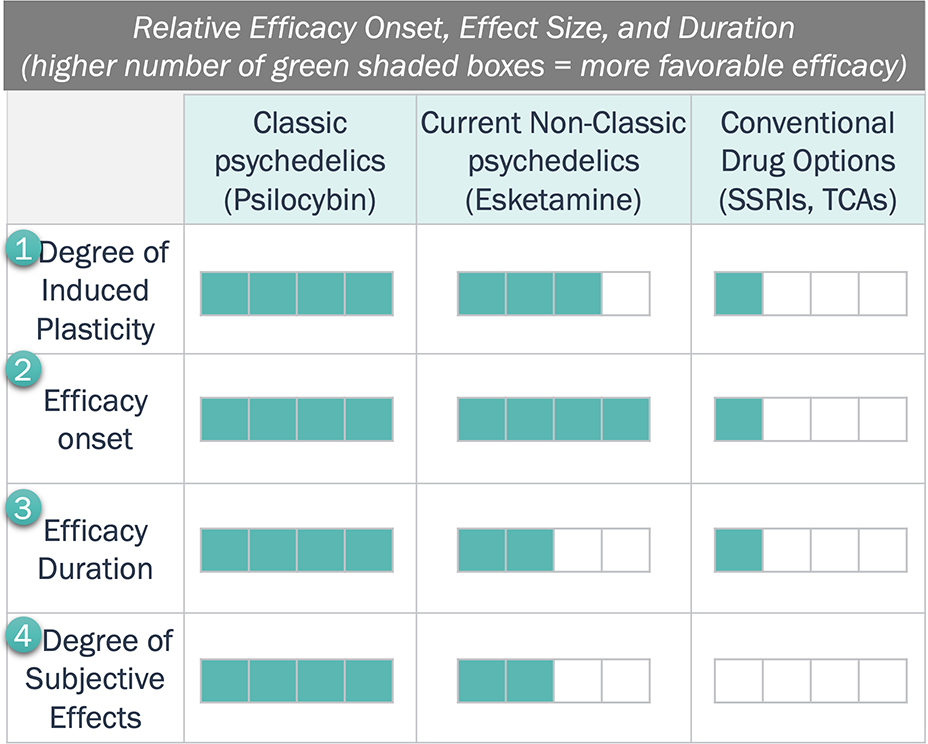
Key Trends Regarding Relative Efficacy
- As “psychoplastogens,” (plasticity-promoting neurotherapeutics) in contrast to conventional options, psychedelics induce a fast, durable promotion of structural and functional neural plasticity. Notably, “classical” psychedelics directly target the 5-HT2A pathway, whereas “non-classic” psychedelics target other receptors, e.g. NMDA.
- Esketamine and psilocybin have efficacy onset within a few hours, while SSRIs may require a few weeks.
- Esketamine and psilocybin are expected to have a longer efficacy duration than SSRIs, with psilocybin being more durable than esketamine. Psilocybin has the potential for a single dose to last for six months, whereas esketamine may require 20+ doses in the same period.
- Subjective effects, i.e., an intense disassociation or perception of altered reality, are a signature feature of psychedelics – one major remaining question is whether the effects of a “trip” are required for therapeutic benefit.
Abbreviations:
SSRI—Serotonin Selective Reuptake Inhibitor.
TCA—Tricyclic Antidepressant.
Sources: (1) Vargas et al. (2021). Frontiers in psychiatry, 12, 727117.
(2) George et al., StatPearls, https://www.ncbi.nlm.nih.gov/books/NBK538165/ (3) Psiuk
et al., (2022). International Journal of Molecular Sciences, 23(19),
11450. (4) company websites (5) ICER 2019.
Faith in the efficacy potential of psychedelics drives development; ~50% of assets in Ph 2 or later have positive POC data with notable remission rates, efficacy onset, and durability
Efficacy Advantages of Psychedelics
- All four Phase 3 assets have FDA breakthrough designation, given early efficacy shown and high unmet need remaining in the indications pursued.
- 18 of 31 assets in Phase 2 or later have positive POC data in their respective indications.

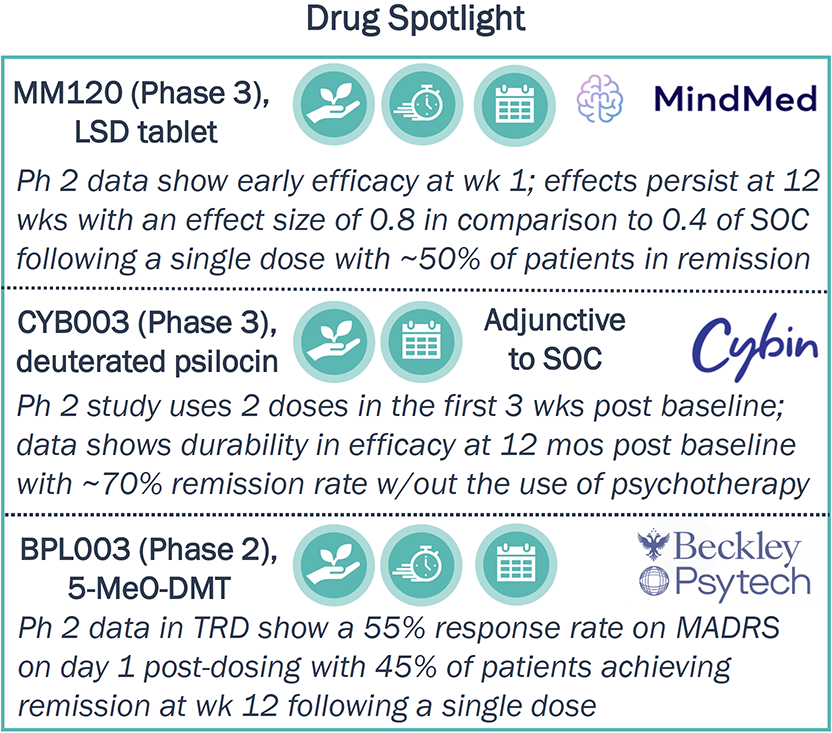
Abbreviations:
POC—Proof of Concept.
SOC—Standard of Care.
wk—week.
mos—months.
5-MeO-DMT—mebufotenin.
MADRS—Montgomery-Åsberg Depression Rating Scale.
TRD—treatment-resistant depression.
Sources: PharmaProjects; Company websites.
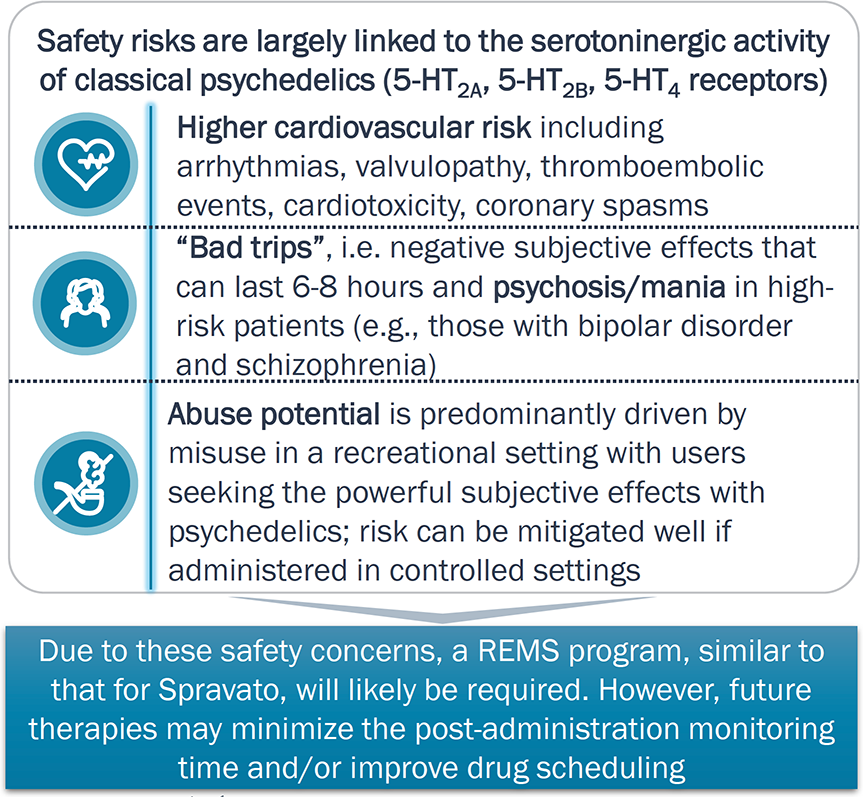
Safety Considerations
The serotonergic activity of classical psychedelics underlie the heightened concerns for their potential side effects and abuse potential, relative to Spravato and conventional drugs
Relative Safety of Psychedelics Compared to Conventional Treatments
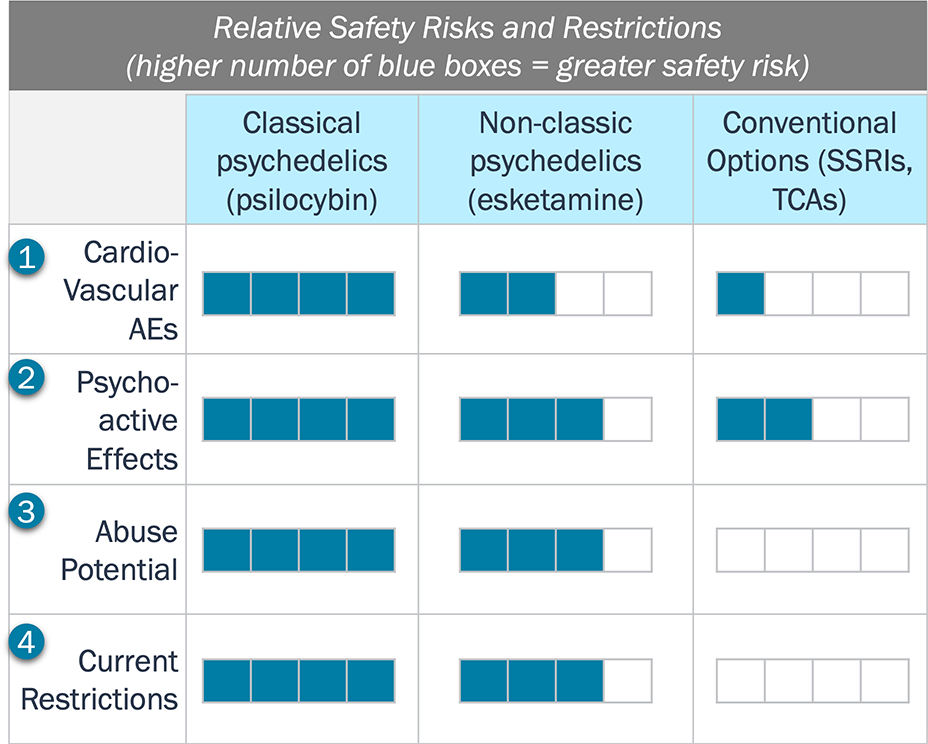
Key Trends Regarding Relative Safety
- In addition to the sympathomimetic risks (e.g., elevated blood pressure for esketamine and hypotension for SSRIs) cardiac toxicities are a concern with classical psychedelics due to their serotoninergic activity (e.g., 5-HT2B).
- The activity at 5-HT2A by classic psychedelics leads to subjective effects and/or risk for mania/psychosis, both which are longer-lasting than esketamine’s dissociative effects; psychedelics may also lower the risk for suicidality (for which SSRIs have a black label) and other nonpsychoactive side effects like weight gain and sexual dysfunction if psychedelics are truly a one-and-done model.
- Despite surveillance data indicating that abuse potential of psychedelics is lower than opioids, regulatory agencies worry that patients may seek the euphoric effects.
- Due to concerns on the psychoactive effects and abuse potential, on classical psychedelics have the highest restrictions (Schedule I) relative to Spravato (Schedule III) and SSRIs (not scheduled).
Abbreviations:
DEA—Drug Enforcement Agency.
Sources: (1) Vargas et al. (2021). Frontiers in psychiatry, 12, 727117.
(2) Wsół, A. (2023). Et al., Pharmacological Reports, 75(6), 1362-1380. (3)
McIntyre, R. S. (2023). Expert Opinion on Drug Safety, 22(10), 881-883. (4)
Yekehtaz et al. (2013), The Journal of Tehran University Heart Center, 8(4),
169. (5) Johnson et al. (2018). The abuse, Neuropharmacology, 142, 143-166.
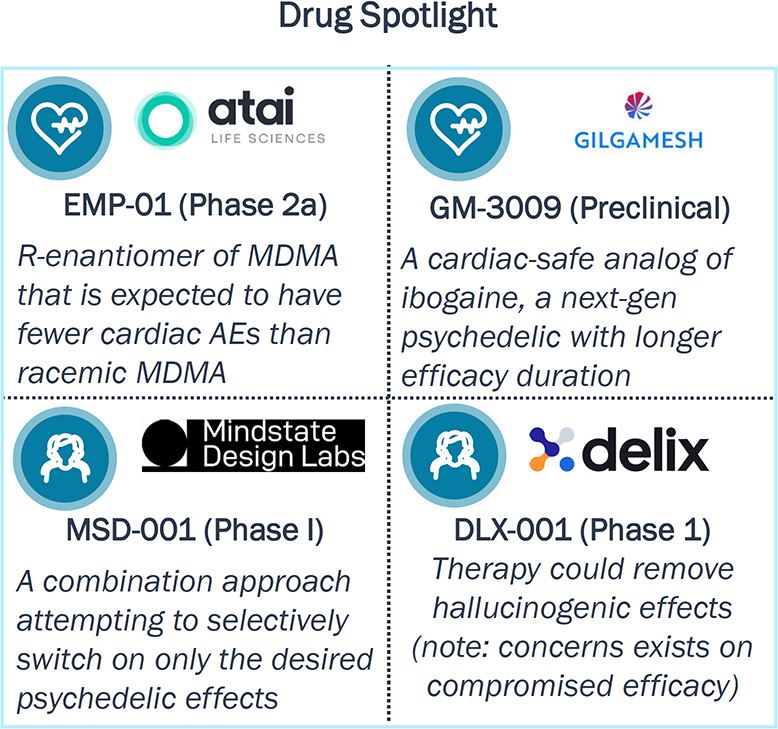
Multiple pipeline agents aim to improve the cardiac and subjective effects linked to classical psychedelics; access is expected to be significantly controlled to minimize abuse potential
Efforts to Improve Safety Profile of Psychedelics
Sources: (1) Vargas et al. (2021). Frontiers in psychiatry, 12, 727117. (2) Wsół, A. (2023). Et al., Pharmacological Reports, 75(6), 1362-1380. (3) McIntyre, R. S. (2023). Expert Opinion on Drug Safety, 22(10), 881-883. (4) Johnson et al. (2018). The abuse, Neuropharmacology, 142, 143-166. (5) Company websites and press releases.
Commercial Outlook
Spravato has set up the infrastructure and provided a roadmap for future launch preparation activities and for broader adoption of psychedelics
Spravato: Trailblazer for Future Psychedelics
CPT codes are five-digit codes used to identify medical
procedures/services and are required for medical billing and reimbursement
Sources: (1) Spravato websites and press releases (2) WSJ, 2024,
“J&J’s Ketamine-Derived Drug Is Taking Off” (3) Pyschedelic
alpha, 2024, Real-World Data Reveals Variability in Access to, and Use of,
Spravato.
2025 could bring significant funding, positive trial results, and policy reform for psychedelics, but questions remain on the drug class’s role in treatment and reimbursement post-approval
Five Key Themes to Watch for in 2025 and Beyond
| 1 | Funding environment |
Will an influx of financing facilitate the post-Lykos comeback for psychedelics?
|
| 2 | Clinical Trial Modifications |
Will companies attempting to address Lykos’ pitfalls succeed?
|
| 3 | Role of Psychotherapy |
How will psychedelics be incorporated into the future treatment paradigm?
|
| 4 | Future Reimbursement |
If future psychedelics are a one and done treatment, how will payers cover these agents?
|
| 5 | Political Climate |
How will the new White House administration impact the development of psychedelics in U.S.?
|
Contact the Authors
|
Send email |
Send email |
Emma Janke, PhD Send email |
|