
January 2026
Oncology’s Red and Blue Oceans: Survival Gains vs. Persistent Gaps
- Bluestar systematically analyzed ~15 of the largest 1L solid tumor settings, comparing current vs. prior standards of care (SoC) in terms of median overall survival (mOS).
- Despite notable progress in select tumor types, more than half of indications still have mOS under 24 months in 1L.
- Tumor types where 1L SoC has improved by >6 months of mOS, which we call "Red Ocean" tumor types, may still have opportunities to improve patient care with better tolerability and addressing needs of poor-responder groups.
- In “Blue Ocean” tumor types where 1L SoC has only improved by <6 months of mOS, there is significant room for improvement with new targets and refined patient selection strategies.
By mapping the evolution of 1L standards of care across major solid tumors, we can systematically identify true areas of unmet need to focus future development.
Challenge
Identifying tumor types with high unmet medical need in oncology requires a multi-dimensional analysis of clinical, regulatory, and market dynamics.
Framework and Approach
Focused on ~15 top solid tumors and relevant sub‑segments*
Documented current vs. prior 1L SoC overall survival (OS)
Categorized tumor types as “Red Ocean” vs. “Blue Ocean” based on magnitude of improvement and absolute OS
*Included patient segments with
estimated 1L U.S.
patients >7,000.
Note: This analysis was conducted November 2025.

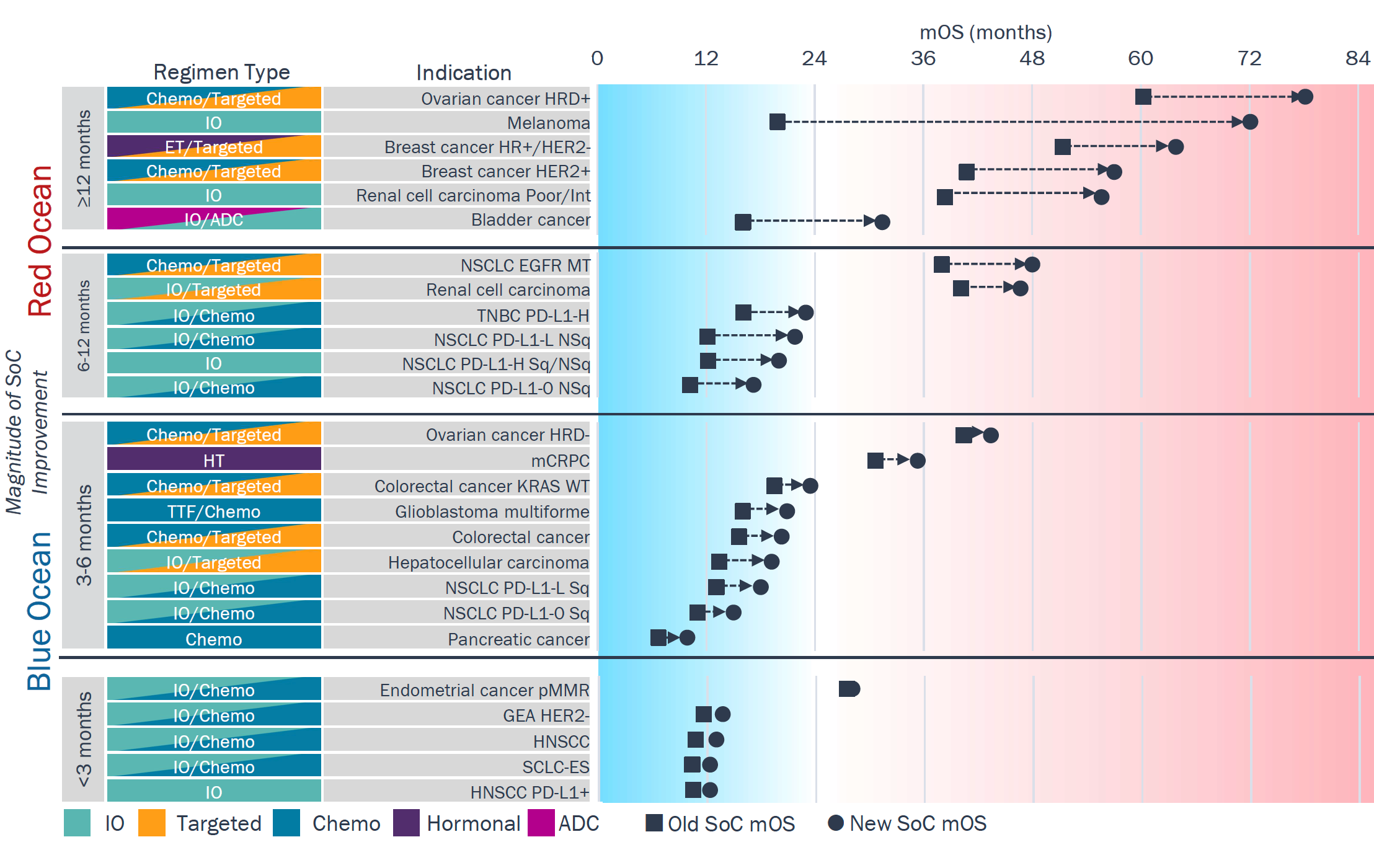
Despite the recent improvement in the 1L SoC across the largest tumor types, more than half still have a mOS of <24 months signifying only incremental benefit in these indications
Median Overall Survival Differential Between Old and New 1L SOC
Abbreviations: NSCLC—Non-small cell lung cancer. TNBC—Triple-negative breast cancer. mCRPC—metastatic castration-resistant prostate cancer. GEA—Gastroesophageal adenocarcinoma. HNSCC—Head and neck squamous cell carcinoma. SCLC-ES—Small-cell lung cancer extensive stage. HRD—Homologous recombination deficient. NSq—Non-squamous. Sq—Squamous. pMMR—Mismatch repair proficient.
"Red Ocean" tumors have seen >6-month gains in 1L mOS, with most now achieving mOS >24 mos.
- ≥12 months 1L SoC gain: Biomarker-driven segments (HER2+ breast, HRD+ ovarian) and very IO-sensitive tumor types (melanoma, RCC) experienced the biggest leaps in OS.
- 6-12 months 1L SoC gain: Most tumor types benefited from IO, but gains remain largely confined to specific subgroups (e.g., non-squamous NSCLC, PD-L1–high TNBC).
"Blue Ocean" tumors have seen <6-month gains in 1L mOS, with most achieving mOS <24 mos.
- 3-6 months 1L SoC gain: Absence of biomarkers to select treatment and no benefit from IO define many of these tumor types (e.g., CRC, mCPRC, PDAC).
- <3 months 1L SoC gain: The introduction of IO did not translate to a significant improvement in these tumor types compared to others.
Red Ocean
Even in "Red Ocean" tumors with strong 1L mOS gains, opportunities remain to better identify poor responders and improve toxicity
Red Ocean: ≥12 Months of Improvement in 1L SoC
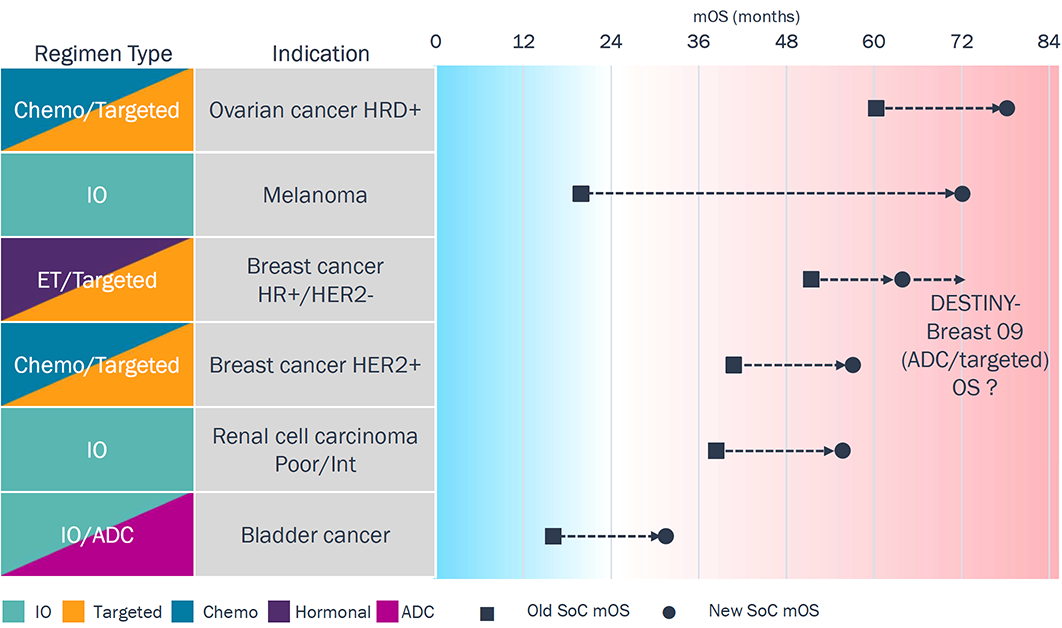
Targeted: ovarian (PARP); HR+/HER2- breast (CDK4/6); HER2+ breast (HER2)
- Melanoma, renal cell carcinoma, and bladder cancer are the only tumor types with broad 1L regimens (no biomarker restriction), all with IO-based SoC; melanoma in particular exemplifies IO’s potential.
- ADCs are beginning to reshape 1L therapy (e.g., EV in bladder
cancer, T-DXd in HER2+ breast), replacing chemo in key
tumor types (e.g., cisplatin in bladder, taxane in HER2+ breast cancer).
- Optimizing ADC use in 1L in terms of duration and combination regimens continues to evolve.
Toxicity with some MoAs (e.g., CTLA-4, ADCs) and sub-groups of refractory patients highlight opportunities to improve tolerability and broaden benefit.
Blue Ocean
"Blue Ocean" tumors with the smallest 1L mOS gains all rely on PD-1 based regimens, suggesting better patient selection and/or novel targets may help increase OS benefit
Blue Ocean: <3 Months of Improvement in 1L SoC
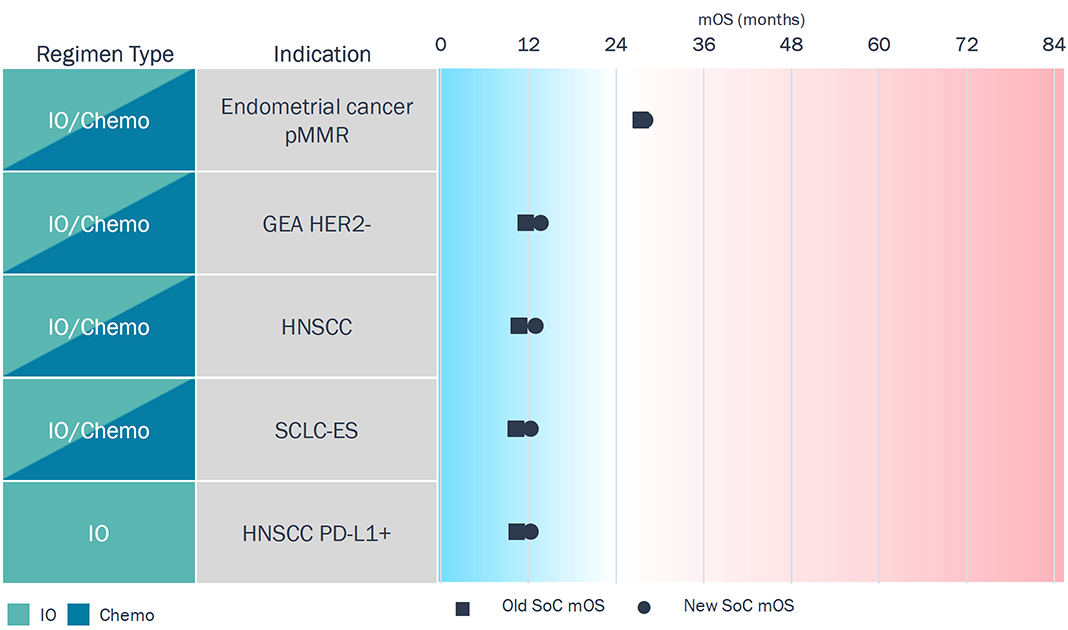
- All tumor types here have IO in 1L, but survival benefit is small; most depend on PD-1 + chemo combination regimens.
- There is limited patient selection — pMMR and HER2- are more representative of the residual patient pool left over after other targeted therapies.
Greater refinement in patient selection (biomarker defined subgroups) and better targets suitable for these tumor types could unlock more meaningful efficacy in these tumor types.
ESMO data highlighted not only the potential but also challenges of developing new targets and patient population selection strategies to address unmet needs
Select ESMO 2025 Abstracts in “Blue Ocean” Tumor Types
Drug (Target)
Bemarituzumab (FGFR2)
Tumor
G/GEJC
Abstract
Bemarituzumab (BEMA) + chemo for advanced or metastatic FGFR2b-overexpressing (G/GEJC): Ph3 FORTITUDE-101 results
Key Takeaways
Early OS benefit seen, but with longer follow-up OS curves have converged. Unique corneal toxicity seen that is reversible. Ph3 FORTITUDE-102 (BEMA + chemo + nivo) also recently failed—unclear if similar toxicity issues persisted.
Drug (Target)
RC118 (CLDN18.2)
Tumor
G/GEJC
Abstract
RC118 (CLDN18.2-targeted ADC) + RC148 (PD-1/VEGF bispecific antibody) or PD-1 for previously treated locally advanced or metastatic G/GEJA
Key Takeaways
ADC + PD-1/VEGF demonstrated ORR 57.1% (vs. 33.3%) and mPFS 7.9mos (vs. 4.3 mos); 1L study for ADC + PD-1/VEGF planned could provide chemo free option for 1L CLDN18.2+.
Drug (Target)
Cadonilimab (PD-1/ CTLA-4)
Tumor
G/GEJC
Abstract
Cadonilimab (Cado) + chemo vs. chemo as 1L G/GEJA : final results Ph3 COMPASSION-15 trial
Key Takeaways
Cadonilimab by Akeso is already approved in China for 1L G/GEJC; final analysis confirms long-term OS benefits, esp. in PD-L1 low-neg groups.
Drug (Target)
Tarlatamab (DLL3xCD3)
Tumor
ES-SCLC
Abstract
Tarlatamab with first-line chemo-IO for ES-SCLC: DeLLphi-303 study
Key Takeaways
mOS of 25.3 mos from start of maintenance for tarlatamab + PD-1 with low grade CRS and ICANS; Ph3 DellPhi-305 is ongoing.
Drug (Target)
Enfortumab vedotin (Nectin-4)
Tumor
HNSCC
Abstract
Enfortumab vedotin plus pembrolizumab as 1L in R/M HNSCC: results from a cohort in EV-202 trial
Key Takeaways
ORR of 39% in 41 patients, mOS not reached; trial enrolled CPS ≥1 patients.
While not at ESMO, there was considerable buzz about Genmab/Merus’s Petosemtamab (EGFRxLGR5 bispecific) in HNSCC which is in Ph3 for 1L PD-L1+ HNSCC; previous Ph2 data showed 63% ORR and 9 mos mPFS.
Source: ESMO 2025 Abstract Titles